eLearning Games
Scroll down to learn how and why medications target these different receptors.
Click through the toggles to learn about the nervous system.
In this section you will learn about the different types of sympathetic and parasympathetic receptors and the medications targeting them. This is a very important concept to understand! A large majority of medications on the market target these two classes of receptors. Master this topic and you are far ahead of your peers.
You will find content to enhance your lectures in the toggles at the top and elearning games to test your knowledge near the bottom of the page.
The nervous system is essentially the body’s electrical wiring and is fundamentally broken down into two parts: the central nervous system (CNS) and the peripheral nervous system (PNS).
Central Nervous System (CNS):
The CNS includes the brain and spinal cord. It serves as the processing center for the nervous system. The brain receives sensory information, processes it, and then decides what needs to be done. The spinal cord transmits signals to and from the brain, and it also carries out some actions on its own (these are known as reflexes).
Peripheral Nervous System (PNS):
The PNS is made up of nerves that branch off from the spinal cord and extend to all parts of the body, including the neck, arms, torso, legs, and internal organs. It carries signals between the CNS and the rest of the body. The PNS is further divided into the somatic nervous system and the autonomic nervous system.
- Somatic Nervous System: This is the part of the PNS that transmits sensory and motor signals to and from the CNS. This system is responsible for voluntary movements, like moving your arm, and it’s involved in reflexes.
- Autonomic Nervous System: This controls involuntary body functions like heartbeat, digestion, and breathing. It’s further divided into the sympathetic and parasympathetic nervous systems.
-
- Sympathetic Nervous System: Often referred to as the “fight or flight” system, the sympathetic nervous system prepares the body for high-intensity, emergency situations. It increases heart rate, dilates pupils, slows digestion, increases blood flow to muscles, and releases glucose for energy. It’s important to remember that prolonged activation of the sympathetic nervous system can have damaging effects on the body, such as weakened immune function and hypertension.
- Parasympathetic Nervous System: This is often referred to as the “rest and digest” system. It’s active during periods of rest and digestion, and it works to conserve energy and restore the body to a calm state. It slows the heart rate, constricts the pupils, stimulates saliva production, promotes digestion, and relaxes muscles. You should understand that an imbalance in the parasympathetic nervous system can lead to conditions such as digestive issues and difficulties with rest and relaxation.
These systems usually work together, and a balance between them is crucial to maintain homeostasis, or stable internal conditions. If one system is overactive or underactive, it can lead to a variety of health issues.
The autonomic nervous system (ANS) is a part of the peripheral nervous system responsible for regulating involuntary body functions, such as heartbeat, blood flow, breathing, and digestion. It functions largely below the level of consciousness and is vital for maintaining homeostasis within the body.
The ANS consists of two primary subsystems: the sympathetic and parasympathetic nervous systems. Let’s take a deeper look into each of them.
Sympathetic Nervous System:
The sympathetic nervous system serves as your emergency response system. It primes your body for action, particularly in situations that require a quick response, eliciting the so-called “fight or flight” response. Here are some of the actions it stimulates:
- It increases heart rate and the force of heart contractions
- It dilates (widens) the airways to enable the lungs to take in more oxygen
- It causes the body to release stored energy
- It dilates pupils, which can improve vision
- It slows down processes that are less important in emergencies, like digestion and urination
- It stimulates the release of adrenaline (epinephrine) and noradrenaline (norepinephrine) to prepare the body for vigorous activity
Many drugs mimic sympathetic nervous system activity, including some asthma medications that dilate the airways and decongestants that constrict blood vessels in the nasal passages.
Parasympathetic Nervous System:
The parasympathetic nervous system, on the other hand, tends to calm the body down and conserve energy, a function sometimes referred to as “rest and digest.” Here’s what it does:
- It slows the heart rate
- It stimulates digestion, including the secretion of digestive enzymes and the movement of food through the digestive system
- It stimulates the body to metabolize food and absorb nutrients
- It contracts the pupils
- It allows the body to relax and recover from the activities that the sympathetic system stimulates
- It promotes the “rest and repair” activities that occur when the body is relaxed
The parasympathetic and sympathetic nervous systems typically have opposing effects. Together, they maintain a balance in the body. This is important for nursing students to understand because various medical conditions, stress levels, and medications can alter this balance, leading to health issues.
Additionally, the ANS also includes the enteric nervous system, sometimes called the “second brain.” This extensive network of neurons lining your gut plays a vital role in controlling digestive function independently of the brain, though it can communicate with the CNS when needed.
In summary, the autonomic nervous system is crucial for maintaining internal stability and responding to changes in the body’s environment. Understanding the ANS can provide you with valuable insights into patient care, particularly when it comes to monitoring vital signs, managing stress responses, and administering medications.
Today we’re going to have a chat about something called the sympathetic nervous system. Now, don’t let the name scare you off. Despite the fancy title, it’s not as complex as you might think. Let’s break it down in the simplest terms possible.
Imagine you’re walking through the woods and suddenly you encounter a bear. Your heart begins to race, your breath quickens, your muscles tighten. This is your body’s “fight or flight” response, a survival mechanism that helps you deal with threats or danger. And the star of this show? You’ve got it – it’s the sympathetic nervous system (SNS).
Our nervous system is kind of like the control panel of our body. It’s a vast network of nerves and cells that send messages to and from our brain and spinal cord to various parts of our bodies. It’s broken down into two main parts: the central nervous system, which is the brain and spinal cord, and the peripheral nervous system, everything else.
The peripheral nervous system is further split into two main components: the somatic nervous system, which controls voluntary movements like waving hello or shaking your head, and the autonomic nervous system, which controls automatic functions like breathing and heartbeat. The autonomic nervous system is then broken down into two sections: the parasympathetic nervous system, which helps the body relax and rest, and our star, the sympathetic nervous system.
The SNS is like the superhero inside us. It’s always on standby, ready to spring into action when we need it most. When there’s danger or any kind of stress, the SNS kicks in and sets off a series of reactions that prepare your body to face it or escape from it.
The effects of the SNS can be felt throughout the body. Here are a few highlights:
1. Heart: When the SNS is activated, your heart beats faster to pump more blood and supply more oxygen to your muscles.
2. Lungs: Your breathing becomes quicker and deeper to take in more oxygen.
3. Eyes: Your pupils dilate to let in more light and improve your sight.
4. Blood vessels: Blood vessels in your muscles expand, while those in your digestive system constrict. This ensures more blood goes to your muscles, so they’re ready for action.
5. Sweat glands: You begin to sweat to cool your body down.
This automatic response is vital for our survival and is what helped our ancestors escape predators and survive in dangerous situations.
But like any superhero, the SNS can face its kryptonite. If the system is activated too often or for too long, it can lead to health issues like high blood pressure, anxiety, and other problems. This is why it’s so important to manage stress and maintain a balanced lifestyle.
Understanding the sympathetic nervous system is vital for your medical career. Not only will it help you comprehend why patients’ bodies are responding in certain ways, but it also forms the foundation of understanding for many treatments and medications. So, take a moment to appreciate your SNS. It’s doing a lot more for you than you might think!
Today, let’s zero in on a chapter of that hefty book – the parasympathetic nervous system (PNS). We’ll keep the language down-to-earth and aim for understanding over scientific jargon. Sound good?
Okay, let’s imagine the human body is a city, bustling with activity 24/7. In this city, we’ve got a management team, known as the autonomic nervous system (ANS), that keeps everything running smoothly without you even noticing. The ANS is split into two main groups: the sympathetic nervous system (SNS) and our main player today, the parasympathetic nervous system (PNS). If the SNS is the emergency response team, dealing with ‘fight or flight’ situations, the PNS is like the leisure and wellness department, focusing on ‘rest and digest’ activities.
Let’s break it down further. The PNS is like the chill-out guru of your body. When you’re safe and full after a delicious meal, it’s the PNS that tells your body to kick back and start digesting. It’s all about conserving energy and maintaining the body’s daily functions.
You see, the PNS uses nerves coming from both the brain (via the cranial nerves) and the lower end of the spinal cord to send messages or ‘orders’ to various parts of the body. The ‘orders’ sent by these nerves can do things like slow the heart rate, stimulate digestion, constrict the pupils, and even help you empty your bladder.
Here’s an everyday example: Imagine you’ve just finished a sumptuous dinner and are settling down for some Netflix. It’s your PNS that swings into action, telling your heart, “Hey buddy, no need to race, let’s slow down.” It also gives your digestive system a nudge, “Time to get to work, my friend. We’ve got nutrients to absorb!” The PNS is the behind-the-scenes orchestrator of this relaxed, restful state.
But how does it accomplish all these tasks? Well, this communication network uses a neurotransmitter, a chemical messenger named acetylcholine (ACh). Acetylcholine is like the PNS’s own personal email system that sends out all the ‘calm down and restore’ commands to the body.
Let’s keep rolling and talk about how the PNS interacts with different parts of your body.
Think of the PNS as the life of a peaceful party, going around and making sure everyone is relaxed and happy. So, what happens when the PNS taps an organ on the shoulder?
1. **Eyes:** When it comes to your peepers, the PNS is like the dimmer switch in a room. It tells your pupils, “Hey, too bright here. Let’s constrict a bit, shall we?” This is especially useful after you’ve been in the dark and then walk into a bright room.
2. **Heart:** Remember how we said the PNS likes to keep things chill? It works its magic on the heart by saying, “Whoa, slow down, buddy!” It lowers your heart rate, making sure you’re not wasting energy when you don’t need to.
3. **Lungs:** The PNS also impacts your breath. It’s like a yoga instructor guiding a deep, calming breath, telling your bronchial muscles, “Let’s take it easy and constrict a bit, no need for that deep panting breath.”
4. **Stomach and Intestines:** Oh, the PNS loves a good meal. After you eat, it tells your stomach and intestines, “Time to get to work, guys! Let’s digest this food and absorb all the nutrients.” It also nudges the gallbladder, “Release that bile! It’ll help break down the fats.”
5. **Liver:** Here, the PNS behaves like a diligent manager overseeing sugar distribution. It signals the liver to store glucose for later use when you might need an energy boost.
6. **Bladder and Rectum:** Now, these are delicate topics, but hey, we all have to answer nature’s call, right? The PNS tells your bladder and rectum, “Alright, time to empty out.” It’s the system responsible for helping you urinate and defecate.
7. **Sex Organs:** Last, but certainly not least, the PNS plays an important role in the ‘rest and reproduce’ part of our lives. In males, it helps induce arousal, and in females, it assists with the lubrication process.
Remember, the PNS isn’t working alone. It’s part of the dynamic duo that forms the autonomic nervous system, balancing out the adrenaline-fueled, high-energy responses of the sympathetic nervous system. Both are necessary and contribute to the harmonious running of the intricate machine that is our body.
So, as you continue your journey, keep in mind that understanding these systems is like understanding the body’s silent language. The more fluent you become, the better you can anticipate and cater to your patients’ needs. But for now, take a deep breath, and let your own PNS do its work. You’ve absorbed a lot of information, and it’s time to rest and digest!
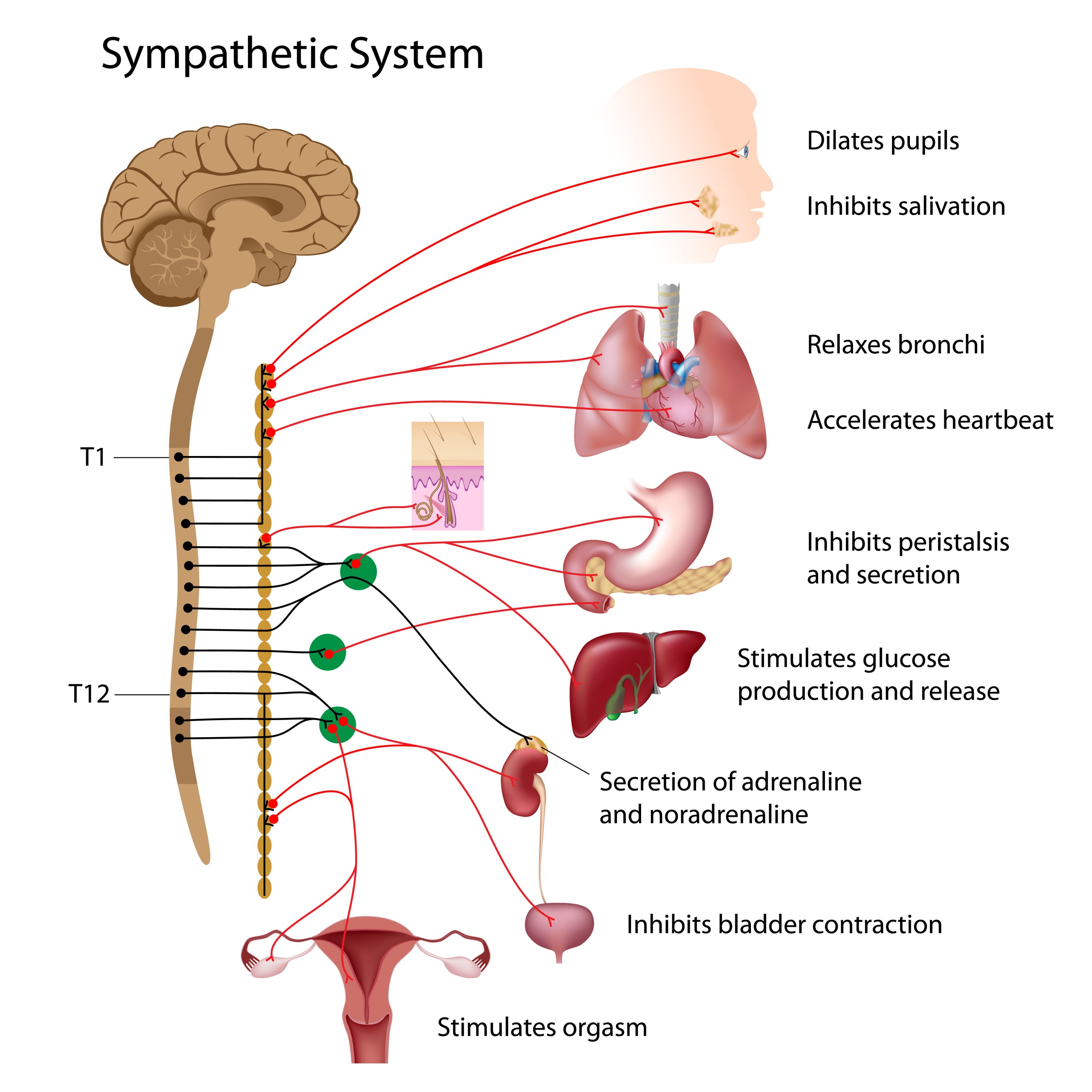
Origination:
The neurons of the sympathetic nervous system originate from the thoracic and lumbar regions of the spinal cord, specifically the intermediolateral nucleus in the spinal cord gray matter. These neurons are known as preganglionic neurons.
Cardiovascular System:
- Heart: The sympathetic nervous system increases the heart rate (tachycardia) and the force of heart contractions (increased contractility), leading to increased cardiac output. It does this by releasing the neurotransmitter norepinephrine which binds to beta-1 receptors on the heart’s pacemaker cells and cardiac muscle cells.
- Blood vessels: Sympathetic activation causes vasoconstriction in most blood vessels, which increases peripheral resistance and thus raises blood pressure. However, in the blood vessels serving the skeletal muscles and the heart, the sympathetic nervous system causes vasodilation, increasing blood flow to these tissues to meet the increased demand during stress or physical exertion.
Respiratory System:
- Bronchial Tubes and Lungs: The sympathetic nervous system causes the bronchial tubes to dilate (bronchodilation), allowing for greater exchange of gases in the lungs, hence more oxygen can be obtained for use by the body’s tissues. It does this by releasing epinephrine which binds to beta-2 receptors on the smooth muscles lining the bronchial tubes.
Eyes:
- Pupils: The sympathetic nervous system causes the pupils to dilate (mydriasis) by contracting the radial muscles of the iris. This allows more light to enter the eye, improving vision under low light conditions, which is useful in response to threats or during “fight or flight” reactions.
Metabolic Processes:
- Liver: The sympathetic nervous system stimulates glycogenolysis (breakdown of glycogen to glucose) and gluconeogenesis (formation of glucose from non-carbohydrate sources) in the liver, thus increasing blood glucose levels to provide more energy for the body during stress or physical
- Fat Cells: The sympathetic nervous system stimulates lipolysis (breakdown of fats to fatty acids) in adipose tissues. These fatty acids can be used as an energy source by many cells in the body.
Endocrine System:
- Adrenal Medulla: The sympathetic nervous system stimulates the adrenal medulla to release the hormones epinephrine (adrenaline) and norepinephrine (noradrenaline) into the bloodstream. These hormones further amplify and extend the effects of the sympathetic nervous system.
Digestive and Urinary Systems:
- Digestive System: Sympathetic activation reduces peristalsis and secretion in the digestive system, which slows down digestion. This allows the body to divert energy to more immediate survival needs during times of stress.
- Urinary System: The sympathetic nervous system reduces urine production by the kidneys during stress or fright situations to avoid the need for elimination while dealing with a stressor. It also stimulates contraction of the internal urethral sphincter to inhibit urination.
Sweat Glands:
- Sweat Production: The sympathetic nervous system stimulates sweat glands to produce sweat, which helps cool the body during periods of stress or high physical exertion.
Immune System:
- Immune Response: Acute sympathetic response may enhance the immune system’s ability to respond to infection or injury. However, chronic stress or over-activation can lead to immune suppression and increased susceptibility to illnesses.
The parasympathetic nervous system essentially acts as the “brakes” of the body, promoting “rest and digest” and “feed and breed” activities. It slows down heart rate, decreases blood pressure, stimulates digestion, and conserves energy for the body.
Origination:
The neurons of the parasympathetic nervous system originate in the brainstem and the sacral (S2-S4) regions of the spinal cord. The cranial nerves involved are the Oculomotor nerve (III), Facial nerve (VII), Glossopharyngeal nerve (IX), and the Vagus nerve (X). The Vagus nerve provides the major parasympathetic output to the body’s organs, supplying about 75% of all parasympathetic fibers.
Cardiovascular System:
- Heart: The parasympathetic nervous system slows down the heart rate (bradycardia) by releasing acetylcholine, which binds to muscarinic receptors on the heart’s pacemaker cells. This decreases the rate and force of cardiac contractions, thus reducing cardiac output.
- Blood vessels: Although the parasympathetic system does not have as much control over blood vessels as the sympathetic system, it does cause some vasodilation, which can lower blood pressure.
Respiratory System:
- Bronchial Tubes and Lungs: The parasympathetic nervous system constricts the bronchial tubes (bronchoconstriction) and reduces respiration rate, allowing for slower, more regular breathing.
Eyes:
- Pupils: The parasympathetic nervous system constricts the pupils (miosis), optimizing them for better focus and near vision.
Metabolic Processes:
- Digestive System: The parasympathetic nervous system stimulates salivation, secretion of digestive enzymes, peristalsis, and the overall activity of the digestive system to process and store food energy.
- Pancreas: The parasympathetic system stimulates the release of insulin from the pancreas, which promotes the uptake and storage of glucose.
Endocrine System:
Adrenal Medulla: Unlike the sympathetic nervous system, the parasympathetic system does not directly stimulate the adrenal medulla.
Urinary System:
- Bladder: The parasympathetic system contracts the bladder and relaxes the internal urethral sphincter, promoting urination.
Reproductive System:
- Sexual Arousal: The parasympathetic nervous system is responsible for sexual arousal in both males (causing erection) and females (causing vaginal lubrication).
Sweat Glands:
Sweat Production: Unlike the sympathetic nervous system, the parasympathetic system does not significantly affect sweat production.
As stated before, the autonomic nervous system (ANS) is a part of the nervous system that controls and regulates the internal organs without any conscious recognition or effort by the individual. It plays a key role in maintaining homeostasis in the body. It’s divided into two primary parts: the sympathetic nervous system and the parasympathetic nervous system.
Adrenergic and cholinergic receptors are important components of the ANS:
-
Adrenergic receptors respond to adrenaline (epinephrine) and noradrenaline (norepinephrine), which are the primary neurotransmitters of the sympathetic nervous system. When these neurotransmitters bind to the adrenergic receptors, it triggers responses like increasing heart rate and blood pressure, dilating the airways, and halting digestion.
-
Cholinergic receptors respond to acetylcholine, the primary neurotransmitter of the parasympathetic nervous system. When acetylcholine binds to these receptors, it initiates responses such as slowing heart rate, constricting the airways, and promoting digestion.
Sympathetic nervous system receptors
- Adrenergic Receptors: These receptors are predominantly involved with the sympathetic nervous system. When they are activated, the body prepares for a “fight or flight” response.
- There are two primary types of adrenergic receptors,
- alpha and beta, and they are further subdivided into alpha1, alpha2, beta1, and beta2.
- Alpha1 receptors are primarily located in the vascular smooth muscle and when activated, they cause vasoconstriction which leads to an increase in blood pressure. Drugs that block these receptors (alpha1 blockers) are often used to treat hypertension.
- Alpha2 receptors are mainly found in the pre-synaptic nerve terminals. Their activation inhibits the release of norepinephrine, thereby reducing sympathetic outflow and reducing blood pressure. Alpha2 agonists are sometimes used in the treatment of hypertension and withdrawal symptoms.
- Beta1 receptors are primarily located in the heart and kidneys. When activated in the heart, they increase heart rate and contractility, thereby increasing cardiac output. In the kidneys, they stimulate the release of renin, which indirectly increases blood pressure. Beta1 blockers are often used in the management of hypertension, heart failure, and arrhythmias.
- Beta2 receptors are mainly found in the smooth muscles of the lungs and the uterus, and also in the liver and skeletal muscles. Activation of these receptors leads to bronchodilation, relaxation of uterine muscles, glycogenolysis in the liver, and enhanced skeletal muscle contraction. Beta2 agonists are typically used in the management of asthma and premature labor.
Parasympathetic nervous system receptors
- Cholinergic Receptors: These receptors are predominantly involved with the parasympathetic nervous system. When activated, they prepare the body for “rest and digest” responses.
- There are two types of cholinergic receptors, muscarinic and nicotinic receptors.
- Muscarinic receptors are found in the smooth muscles, heart, and glands. When activated, they decrease heart rate and force of contraction (M2 subtype), stimulate glandular secretions, and cause smooth muscle (including bronchial) constriction and increased gut motility (M3 subtype). Drugs that block these receptors (antimuscarinics) can be used to treat various conditions like overactive bladder, COPD, and bradycardia.
- Nicotinic receptors are located in the autonomic ganglia and skeletal muscle. Activation of these receptors in the autonomic ganglia can stimulate both sympathetic and parasympathetic responses, depending on the predominant tone in that organ. In the skeletal muscle, their activation leads to muscle contraction. Nicotinic receptor blockers can be used in surgery for muscle relaxation and in smoking cessation therapies.
Cholinergic agonists, also known as parasympathomimetic agents, are a class of drugs that mimic the action of acetylcholine, a major neurotransmitter in the body. Acetylcholine is the primary neurotransmitter used at the neuromuscular junction, in the autonomic ganglia, and in a variety of central nervous system synapses. Therefore, cholinergic agonists can have a wide range of effects based on their mechanism of action and their target receptors.
The primary function of cholinergic agonists is to bind to and activate the cholinergic receptors. There are two types of cholinergic receptors in the body: muscarinic receptors and nicotinic receptors. Muscarinic receptors are predominantly found in the parasympathetic nervous system, heart, smooth muscles, and various glands. Nicotinic receptors are found in the autonomic ganglia, the central nervous system, and skeletal muscle.
- Muscarinic agonists:
These are cholinergic agonists that primarily act on the muscarinic receptors. They cause effects similar to those of parasympathetic nerve stimulation, which is often referred to as the “rest and digest” system. Therefore, these agents can slow the heart rate, stimulate gland secretions, increase smooth muscle activity in the gastrointestinal tract, and contract the muscles of the pupil (causing miosis).- Examples of muscarinic agonists include pilocarpine, which is used to treat glaucoma by reducing intraocular pressure, and bethanechol, which is used to stimulate the bladder and gastrointestinal tract in cases of postoperative and postpartum nonobstructive urinary retention and gastric atony.
- Nicotinic agonists
These cholinergic agonists primarily act on the nicotinic receptors. Their effects can be both stimulatory and inhibitory, depending on the specific type of nicotinic receptor they interact with and their location in the body.- Nicotine, for instance, is a well-known nicotinic agonist. It is used therapeutically in smoking cessation products to help with nicotine addiction. It stimulates the release of various neurotransmitters in the brain, providing the pleasurable sensations that can lead to dependence.
It’s important to note that the effect of a cholinergic agonist can be beneficial or detrimental, depending on the context. While they can be used therapeutically to treat a variety of conditions, they can also cause undesirable effects if used improperly or in excessive amounts. For example, an overdose of a cholinergic agonist can lead to a cholinergic crisis, which can cause symptoms such as excessive salivation, bronchoconstriction, bradycardia, and even potentially fatal respiratory failure.
Moreover, certain diseases or conditions can alter the body’s response to cholinergic agonists. For instance, in patients with myasthenia gravis, an autoimmune disorder that affects neuromuscular transmission, cholinergic agonists like pyridostigmine are used to increase the amount of acetylcholine available at the neuromuscular junction, improving muscle strength.
In summary, cholinergic agonists are a diverse group of drugs that can have a wide variety of effects on the body. They are used in the treatment of many conditions, but their use needs to be carefully managed due to the potential for side effects and interactions with other drugs or conditions.
Cholinergic antagonists, also known as anticholinergic agents, are a class of drugs that inhibit the action of acetylcholine, a neurotransmitter that plays a crucial role in transmitting signals across nerve synapses. These drugs work by blocking the binding of acetylcholine to its receptors, effectively hindering its action.
Just as with cholinergic agonists, cholinergic antagonists are divided based on the type of cholinergic receptors they act upon: muscarinic receptors and nicotinic receptors.
- Muscarinic Antagonists:
Muscarinic antagonists, also known as antimuscarinic agents, inhibit the action of acetylcholine on muscarinic receptors. These receptors are predominantly found in the parasympathetic nervous system, heart, smooth muscles, and various glands.- Antimuscarinic agents have a range of applications. They can be used to increase heart rate, reduce gland secretions, relax smooth muscles, and dilate the pupils (causing mydriasis). They are often used in the treatment of a variety of conditions including
- overactive bladder
- gastrointestinal spasms
- COPD
- Parkinson’s disease
- certain types of poisoning.
- Antimuscarinic agents have a range of applications. They can be used to increase heart rate, reduce gland secretions, relax smooth muscles, and dilate the pupils (causing mydriasis). They are often used in the treatment of a variety of conditions including
Examples of these drugs include atropine, which is used to increase heart rate in bradycardia and as an antidote for certain types of poisonings; scopolamine, used for motion sickness and postoperative nausea and vomiting; and tolterodine or oxybutynin, used in the management of overactive bladder.
- Nicotinic Antagonists:
Nicotinic antagonists inhibit the action of acetylcholine on nicotinic receptors. Nicotinic receptors are found in the autonomic ganglia, the central nervous system, and skeletal muscle.- Nicotinic antagonists are further divided into two categories: ganglionic blockers and neuromuscular blockers. Ganglionic blockers, like trimethaphan, are rarely used today due to their severe side effects, but they have historically been used to manage hypertension. Neuromuscular blockers, like rocuronium and vecuronium, are used to induce muscle relaxation during surgeries.
Just like their agonist counterparts, cholinergic antagonists can also have adverse effects. Overdose or chronic use can lead to anticholinergic syndrome, characterized by symptoms such as
- blurred vision
- dry mouth
- constipation
- urinary retention
- confusion
- hyperthermia.
In addition, people with certain conditions, such as glaucoma, benign prostatic hyperplasia, or bowel obstruction, need to use anticholinergic drugs with caution because of the potential worsening of symptoms.
In summary, cholinergic antagonists are a class of drugs that counteract the action of acetylcholine. They can have a wide range of effects depending on which type of receptor they interact with and where in the body these receptors are located. They are used in the management of a variety of conditions, but their use must be carefully monitored due to the potential for side effects and interactions with other drugs or conditions.
Adrenergic agonists, also known as sympathomimetics, are a class of drugs that stimulate the adrenergic receptors in the sympathetic nervous system. They mimic the action of the adrenal hormones, specifically epinephrine (adrenaline) and norepinephrine (noradrenaline), which are the body’s natural fight-or-flight hormones.
Adrenergic receptors are categorized into alpha-1, alpha-2, and beta-1, beta-2 receptors. Each type is distributed differently throughout the body, and each has different effects upon activation.
- Alpha-1 Agonists:
Alpha-1 adrenergic agonists stimulate the alpha-1 receptors, predominantly found on vascular smooth muscle, leading to vasoconstriction. This increases blood pressure and is used in treating conditions like hypotension or nasal congestion. Phenylephrine is an example of an alpha-1 agonist, often used as a decongestant. - Alpha-2 Agonists:
Alpha-2 adrenergic agonists, such as clonidine and dexmedetomidine, act on alpha-2 receptors located in the central nervous system and peripheral vascular smooth muscle. When stimulated, these receptors inhibit the release of norepinephrine, reducing sympathetic outflow. This leads to decreased blood pressure and is often used in treating hypertension or withdrawal symptoms from opioids. - Beta-1 Agonists:
Beta-1 adrenergic agonists stimulate the beta-1 receptors primarily located in the heart. This leads to increased heart rate and contractility. Dobutamine, for example, is a beta-1 agonist used in heart failure or cardiogenic shock to increase cardiac output. - Beta-2 Agonists:
Beta-2 adrenergic agonists act on the beta-2 receptors found in the lungs, leading to bronchodilation. They are typically used in the treatment of asthma and other chronic obstructive pulmonary diseases (COPD). Examples include albuterol and salmeterol. - Nonselective Adrenergic Agonists
Some adrenergic agonists, like epinephrine, are nonselective and stimulate multiple types of adrenergic receptors. Epinephrine, for example, can increase heart rate, cause vasoconstriction, and induce bronchodilation. It is often used in emergencies such as anaphylaxis or cardiac arrest.
While adrenergic agonists have many therapeutic uses, their use should be carefully monitored. Overuse or misuse can lead to
- high blood pressure
- tachycardia
- palpitations
- anxiety
- moreover, they can also have significant interactions with other drugs.
In summary, adrenergic agonists are a class of drugs that stimulate the adrenergic receptors in the body, mimicking the action of adrenaline and noradrenaline. Their effects vary widely depending on which type of adrenergic receptor they stimulate, and they are used in the treatment of a variety of conditions, from heart failure to asthma to hypotension.
Adrenergic antagonists, also known as sympatholytics, are a class of drugs that inhibit the action of the adrenergic neurotransmitters, specifically epinephrine (adrenaline) and norepinephrine (noradrenaline). They work by blocking the binding of these neurotransmitters to their receptors, effectively reducing their action.
Similar to adrenergic agonists, adrenergic antagonists can be divided based on the type of adrenergic receptor they act upon: alpha and beta receptors. These categories can be further divided into alpha-1, alpha-2, beta-1, and beta-2 antagonists.
- Alpha-1 Antagonists:
Alpha-1 adrenergic antagonists, also known as alpha-1 blockers, inhibit the action of the neurotransmitters on the alpha-1 receptors, which are predominantly found on vascular smooth muscle. This action leads to vasodilation and a decrease in blood pressure. These agents are often used in treating conditions such as hypertension and benign prostatic hyperplasia (an enlarged prostate). Examples include doxazosin and tamsulosin. - Alpha-2 Antagonists:
Alpha-2 adrenergic antagonists block the alpha-2 receptors, which are located in the central and peripheral nervous system. Alpha-2 antagonists are not commonly used in clinical practice because their action tends to increase sympathetic outflow, leading to an increase in blood pressure. However, one of the alpha-2 antagonists, yohimbine, is sometimes used for the treatment of erectile dysfunction. - Beta-1 Antagonists:
Beta-1 adrenergic antagonists, also known as beta-1 blockers, inhibit the action on the beta-1 receptors that are primarily located in the heart. This leads to a decrease in heart rate and contractility, reducing the work of the heart. They are commonly used in the treatment of conditions such as hypertension, angina, heart failure, and irregular heart rhythms. Examples include metoprolol and atenolol. - Beta-2 Antagonists:
Beta-2 adrenergic antagonists block the beta-2 receptors, primarily located in the lungs. While they have theoretical applications, their use can potentially lead to bronchoconstriction, so they are rarely used in clinical practice. However, nonselective beta blockers (which block both beta-1 and beta-2 receptors), like propranolol, can inadvertently block beta-2 receptors, potentially causing issues in patients with respiratory conditions like asthma. - Nonselective Adrenergic Antagonists:
Nonselective adrenergic antagonists block multiple types of adrenergic receptors. For example, labetalol is an antagonist of alpha-1, beta-1, and beta-2 receptors and is used in managing hypertension, especially in pregnancy.
Adrenergic antagonists are generally safe but must be used with caution due to potential side effects like hypotension, bradycardia, fatigue, and, in the case of nonselective beta blockers, potential bronchoconstriction. Sudden withdrawal of these drugs, especially in the treatment of heart disease, can lead to rebound hypertension or angina, so it’s important to gradually decrease the dose under medical supervision.
In summary, adrenergic antagonists are a class of drugs that inhibit the adrenergic neurotransmitters, adrenaline and noradrenaline, reducing their effects on the body. They are used in the treatment of various conditions, including hypertension, heart failure, and benign prostatic hyperplasia, among others. Their use must be carefully monitored due to potential side effects and interactions with other drugs.
Muscarinic Receptors
Muscarinic receptors are a type of receptor in the body that are part of the larger class of G protein-coupled receptors (GPCRs). They play an important role in the functioning of the parasympathetic nervous system, and their activation leads to a variety of physiological responses. These receptors are named after the compound muscarine, which binds to and activates them, unlike the other class of cholinergic receptors, the nicotinic receptors, which respond to nicotine.
Types and Locations
Five subtypes of muscarinic receptors have been identified to date, designated M1 to M5.
- M1 receptors are mainly found in the brain, autonomic ganglia, and stomach. They play a role in cognitive functions like learning and memory.
- M2 receptors are predominantly located in the heart and smooth muscle. They help in reducing heart rate and contractility.
- M3 receptors can be found in many places, including the smooth muscles of the lungs and gastrointestinal tract, exocrine glands, and endothelial cells. They stimulate glandular secretion and smooth muscle contraction.
- M4 receptors are mainly found in the brain, and they are thought to play a role in controlling dopamine release.
- M5 receptors are least understood among all. They are mainly found in the brain and are involved in enhancing dopamine release.
Functions
Each type of muscarinic receptor has a different function depending on its location, but in general, they are involved in maintaining homeostasis in the body and mediating the “rest and digest” response. They aid in slowing heart rate, increasing glandular secretion, promoting digestion, constricting the pupils, and promoting urinary function.
Targeting by Drugs
Because of their various roles in the body, muscarinic receptors are important targets for many drugs. Drugs can act as agonists (activating the receptor) or antagonists (blocking the receptor).
- Agonists: Drugs such as pilocarpine and cevimeline are used to treat dry mouth and dry eyes, as they stimulate the M3 receptors in the salivary and lacrimal glands to increase secretion. Other drugs, like bethanechol, stimulate M3 receptors in the bladder, helping it to empty.
- Antagonists: These are often referred to as anticholinergics or parasympatholytics. They include drugs like atropine and scopolamine. Atropine, for instance, blocks M2 receptors in the heart, increasing heart rate, and blocks M3 receptors in the eye, leading to pupil dilation. Scopolamine, used to treat motion sickness, primarily acts on the central nervous system.
Potential Side Effects and Considerations
Given the widespread nature of muscarinic receptors, drugs that target these receptors can often have wide-ranging side effects. For instance, anticholinergic drugs can cause (very important to know!)
- dry mouth
- blurred vision
- constipation
- urinary retention
- cognitive impairment.
Understanding the role and functioning of muscarinic receptors is crucial, as many common drugs act on these receptors. This knowledge will enable you to understand the mechanisms of action of these drugs, predict their potential side effects, and provide better care for patients.
Always bear in mind the parasympathetic nervous system’s role, remember the effects of muscarinic receptors on various organ systems, and consider the potential for drug interactions in patients who are taking multiple medications.
Nicotinic Receptors
Nicotinic receptors are a type of cholinergic receptor that respond to the neurotransmitter acetylcholine. They are named after nicotine, a compound in tobacco that also binds to these receptors, stimulating them. Unlike muscarinic receptors, which are G-protein coupled, nicotinic receptors are ligand-gated ion channels.
Types and Locations
There are two main types of nicotinic acetylcholine receptors (nAChRs) based on their location: neuronal (nN) and muscular (nM).
- Neuronal nicotinic receptors (nAChR-nN) are predominantly found in the brain and autonomic ganglia. They are composed of various combinations of alpha and beta subunits (α2-α10, β2-β4), and the composition of these subunits determines the receptor’s specific properties.
- Muscular nicotinic receptors (nAChR-nM) are located at the neuromuscular junction, where the nerves meet the muscles. These receptors are composed of two α1 subunits and one each of β1, δ, and ε (or γ in embryonic muscle).
Functions
Nicotinic receptors play vital roles in a variety of physiological processes:
- At the neuromuscular junction, the activation of nAChR-nM by acetylcholine leads to muscle contraction.
- In the brain, nAChR-nN are involved in various functions, such as memory, cognition, and arousal.
- In the autonomic ganglia, they mediate the transmission of signals from pre-ganglionic neurons to post-ganglionic neurons, playing a critical role in both the sympathetic and parasympathetic nervous systems.
Targeting by Drugs
Many drugs target nicotinic receptors, acting as either agonists (activating the receptor) or antagonists (blocking the receptor).
- Agonists: Nicotine, the well-known active component of tobacco, is a stimulant that primarily acts as an agonist at nicotinic receptors in the brain, leading to increased arousal, memory, and cognition. However, it also has addictive properties. Other drugs, like varenicline (Chantix), are used to aid smoking cessation by partially stimulating the receptors that respond to nicotine, thereby reducing cravings and withdrawal symptoms.
- Antagonists: These include drugs like tubocurarine and vecuronium, which are non-depolarizing muscle relaxants used in anesthesia. They work by blocking the nAChR-nM at the neuromuscular junction, preventing muscle contraction. Another antagonist, mecamylamine, blocks nAChR-nN in the autonomic ganglia and has been used in the past to treat hypertension.
Potential Side Effects and Considerations
The effects of drugs targeting nicotinic receptors can be widespread, given the distribution of these receptors in the body.
Potential side effects can include
- muscle weakness or paralysis (for drugs acting on the neuromuscular junction)
- various effects on the brain, such as memory impairment or addiction (for drugs acting on neuronal nicotinic receptors).
Understanding the functions of nicotinic receptors is vital as it affects many aspects of patient care. For example, the use of muscle relaxants during anesthesia or the treatment of nicotine addiction. Being aware of these receptors’ mechanisms of action helps predict potential side effects and manage drug interactions effectively in patients taking multiple medications.
Alpha-1 Receptors
Alpha-1 (α1) receptors are a type of adrenergic receptor that is responsive to the catecholamines epinephrine (adrenaline) and norepinephrine (noradrenaline). These receptors are part of the G-protein-coupled receptor (GPCR) family, and they primarily act through the Gq protein to stimulate the phospholipase C (PLC) pathway.
Types and Locations
Alpha-1 receptors have three main subtypes: α1A, α1B, and α1D. They are expressed throughout the body but are particularly concentrated in certain locations:
- α1A receptors are primarily found in the prostate, blood vessels, and heart. They play a critical role in smooth muscle contraction.
- α1B receptors are abundant in the spleen, liver, and kidneys, and contribute to the regulation of various functions in these organs.
- α1D receptors are mostly located in the heart, blood vessels, and brain, and participate in modulating cardiovascular responses.
Functions
Alpha-1 receptors have multiple physiological roles, mainly revolving around vasoconstriction and the contraction of smooth muscle:
- Vasoconstriction: When activated by epinephrine or norepinephrine, α1 receptors cause the smooth muscles lining blood vessels to contract, leading to vasoconstriction. This results in an increase in systemic vascular resistance and blood pressure.
- Smooth Muscle Contraction: Alpha-1 receptors also regulate the contraction of smooth muscle in other parts of the body, such as the genitourinary tract and gastrointestinal tract. For example, their activation in the urinary bladder’s neck and urethra promotes urinary continence.
- Glycogenolysis and Gluconeogenesis: α1 receptors in the liver participate in the regulation of blood glucose levels by promoting glycogenolysis and gluconeogenesis.
Targeting by Drugs
The alpha-1 receptor is a critical target for many drugs, which can act as either agonists (activating the receptor) or antagonists (blocking the receptor).
- Agonists: Drugs like phenylephrine and oxymetazoline are α1 agonists, and are often used as decongestants. They induce vasoconstriction in the nasal blood vessels, reducing blood flow and alleviating nasal congestion.
- Antagonists: Drugs such as doxazosin, terazosin, and tamsulosin are α1 antagonists, or alpha blockers. They are used in managing hypertension and benign prostatic hyperplasia (BPH). By blocking the action of α1 receptors, these drugs promote vasodilation, reducing blood pressure, and relax the smooth muscle in the prostate, alleviating urinary symptoms.
Potential Side Effects and Considerations
Drugs acting on α1 receptors can have side effects due to their systemic actions. Alpha blockers, for instance, can cause
- orthostatic hypotension (a sudden drop in blood pressure when standing up)
- dizziness
- nasal congestion.
α1 agonists can lead to
- hypertension (high blood pressure)
- reflex bradycardia (a slower heart rate).
Understanding the role and functions of α1 receptors is essential, as it impacts the clinical application of several important drug classes. This knowledge will enable you to predict potential side effects, manage drug interactions, and ensure the safe and effective use of medications in patient care.
Alpha-2 Receptors
Alpha-2 (α2) receptors are a subtype of adrenergic receptors, a class of G-protein-coupled receptors (GPCRs) that respond to the catecholamines epinephrine (adrenaline) and norepinephrine (noradrenaline). Unlike α1 receptors, which primarily induce cellular responses via the Gq protein, α2 receptors typically act through the Gi protein, inhibiting adenylate cyclase and decreasing intracellular levels of cyclic adenosine monophosphate (cAMP).
Types and Locations
There are three main subtypes of α2 receptors: α2A, α2B, and α2C. They are found in various tissues and organs:
- α2A receptors are largely present in the central nervous system (brain and spinal cord), and also found in the kidney and pancreas.
- α2B receptors are mainly located in the blood vessels.
- α2C receptors are predominantly found in the brain, with lesser quantities in the heart and kidneys.
Functions
The α2 receptors have multiple functions throughout the body:
- Presynaptic inhibition: In the central and peripheral nervous systems, α2 receptors are often found presynaptically (on the neuron releasing the neurotransmitter). When activated, they inhibit the release of neurotransmitters, including norepinephrine and acetylcholine, thereby reducing neural activity. This is called a negative feedback mechanism.
- Control of Insulin Release: α2 receptors in the pancreas inhibit insulin release, thereby playing a role in the regulation of blood glucose levels.
- Vasoconstriction: Unlike their primary role of inhibiting cellular activity, α2B receptors on the smooth muscle of blood vessels cause vasoconstriction when activated.
Targeting by Drugs
Drugs can target α2 receptors as either agonists (activating the receptor) or antagonists (blocking the receptor).
- Agonists: Drugs like clonidine and dexmedetomidine are α2 agonists used for their hypotensive and sedative effects. By activating α2 receptors in the brain, they reduce sympathetic outflow, leading to decreased heart rate and blood pressure. They are used in managing hypertension, opioid withdrawal symptoms, and as adjuncts in anesthesia.
- Antagonists: Yohimbine and mirtazapine are examples of α2 antagonists. Yohimbine is used occasionally to treat erectile dysfunction, while mirtazapine is used as an antidepressant, sleep aid, and appetite stimulant.
Potential Side Effects and Considerations
Because α2 receptors are widely distributed, drugs targeting these receptors can have diverse side effects. For instance, α2 agonists can cause
- dry mouth
- sedation
- and bradycardia (slow heart rate).
- Abrupt discontinuation of these drugs can lead to a “rebound” effect, with symptoms of rapid heart rate, sweating, and anxiety.
On the other hand, α2 antagonists can lead to
- increased heart rate and blood pressure.
Understanding the function of α2 receptors is crucial. Their roles in various physiological processes mean that drugs targeting these receptors have many therapeutic applications. Having this knowledge will allow you to predict potential drug effects and side effects, and to effectively manage patient care.
Beta-1 Receptors
Beta-1 (β1) receptors are one of three types of beta-adrenergic receptors. As part of the larger family of G-protein-coupled receptors (GPCRs), they are primarily stimulated by the catecholamines, epinephrine (adrenaline) and norepinephrine (noradrenaline). Upon activation, β1 receptors typically stimulate the Gs protein, leading to the activation of adenylyl cyclase, which in turn increases the levels of cyclic adenosine monophosphate (cAMP) within the cell.
Locations
The β1 receptors are predominantly found in the heart and kidneys. In the heart, they are located in the sinoatrial node (the heart’s natural pacemaker), atria, and ventricles. In the kidneys, they are found in the juxtaglomerular cells.
Functions
The β1 receptors have several critical functions:
- Cardiac Function: In the heart, β1 receptors are involved in regulating the rate and strength of the heartbeat. Their activation increases the heart rate (positive chronotropic effect), enhances the speed of electrical conduction through the heart (positive dromotropic effect), and increases the force of the heart’s contraction (positive inotropic effect).
- Renin Release: In the kidneys, β1 receptors regulate the release of renin, an enzyme involved in the regulation of blood pressure and fluid balance. (This concept is very important and you will learn more about it in the cardiac drugs section)
Targeting by Drugs
Drugs can act on β1 receptors as either agonists (activating the receptor) or antagonists (blocking the receptor).
- Agonists: Drugs like dobutamine and isoproterenol are β1 agonists. Dobutamine is often used in heart failure or cardiogenic shock to increase the heart’s contractility and output without substantially increasing heart rate.
- Antagonists: Beta blockers, such as metoprolol and atenolol, are drugs that primarily block β1 receptors. They are used in the management of a variety of cardiovascular conditions, including hypertension, angina, and heart failure. By blocking the β1 receptors, these drugs reduce heart rate, decrease the force of heart muscle contraction, and reduce renin release from the kidneys, collectively lowering blood pressure and reducing the heart’s oxygen demand.
Potential Side Effects and Considerations
The use of drugs targeting β1 receptors can have several side effects. For instance, β1 antagonists can cause
- bradycardia (slow heart rate)
- hypotension (low blood pressure)
- fatigue (exercise intolerance due to forced lowering of heart rate thus oxygen doesn’t circulate to tissues as quickly as they normally would with increased breathing with exercise)
- Masking of hypoglycemic symptoms (this is asked all of the time on tests!)
- in some cases, may worsen symptoms of heart failure or asthma
β1 agonists, on the other hand, can cause
- tachycardia (rapid heart rate)
- palpitations
- rise in blood pressure
As a nursing student, understanding the role of β1 receptors and their associated drugs is essential. Such understanding allows you to anticipate the potential therapeutic effects and side effects of these medications, manage drug interactions, and provide better care for your patients, particularly those with cardiovascular disorders.
Beta-2 Receptors
Beta-2 (β2) receptors are a subtype of beta-adrenergic receptors, part of the larger family of G-protein-coupled receptors (GPCRs). They are primarily responsive to the catecholamines epinephrine (adrenaline) and norepinephrine (noradrenaline). Like β1 receptors, β2 receptors primarily act through the Gs protein to stimulate adenylyl cyclase and increase cyclic adenosine monophosphate (cAMP) levels in the cell.
Locations
Beta-2 receptors are widely distributed throughout the body but are particularly prominent in the lungs, vascular smooth muscle, skeletal muscle, and liver.
Functions
The β2 receptors have several vital functions:
- Bronchodilation: In the lungs, the activation of β2 receptors relaxes the smooth muscles of the bronchi and bronchioles, leading to bronchodilation. This helps increase airflow and makes it easier to breathe.
- Vasodilation: In blood vessels, activation of these receptors causes vasodilation, which reduces vascular resistance and lowers blood pressure.
- Glycogenolysis and Gluconeogenesis: In the liver and skeletal muscles, β2 receptors regulate glycogenolysis (the breakdown of glycogen into glucose) and gluconeogenesis (the production of glucose from non-carbohydrate sources). Both processes increase blood glucose levels.
Targeting by Drugs
Drugs can act on β2 receptors as either agonists (activating the receptor) or antagonists (blocking the receptor).
- Agonists: Drugs like albuterol, salbutamol, and formoterol are β2 agonists primarily used in the management of respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). By activating β2 receptors in the lungs, these drugs cause bronchodilation, relieving symptoms like shortness of breath and wheezing.
- Antagonists: While there are few clinical applications for β2 antagonists, non-selective beta blockers like propranolol do block β2 receptors alongside β1. This can lead to bronchoconstriction, which is why non-selective beta blockers are generally avoided in patients with asthma or COPD.
Potential Side Effects and Considerations
Drugs acting on β2 receptors can have side effects due to their systemic action. β2 agonists can lead to tachycardia (fast heart rate), palpitations, muscle tremors, and in some cases, hypokalemia (low potassium levels in the blood). As mentioned, non-selective beta blockers can cause bronchoconstriction, which can exacerbate respiratory diseases.
Understanding β2 receptors is essential due to their relevance in respiratory and metabolic disorders. This knowledge helps predict potential drug effects and side effects, manage drug interactions, and provide appropriate patient education about the use of these medications.
Terms to Know – Sympathetic and Parasympathetic Nervous Systems
Pharmacodynamics refers to the study of the biochemical and physiological effects of drugs on the body and their mechanisms of action. It involves analyzing how a drug interacts with its target, such as receptors or enzymes, to exert its therapeutic or toxic effects. It is often defined as “What the drug does to the body.”
Pharmacokinetics also refers to the movement of a drug throughout the body.
Each phase of pharmacokinetics – absorption, distribution, metabolism, and excretion – involve drug movement through membranes. Drugs pass through membranes in a variety of ways but three are most important – Direct penetration, facilitated diffusion, and active transport.
- Direct penetration is the process by which drugs cross biological membranes, such as the cell membrane’s phospholipid bilayer, by passive diffusion without the need for membrane transporters, channels, or the expenditure of energy. This is the most common method of drug transport as most drugs are too large to fit through channels and pores and most lack a transport system.
Drugs that are lipid soluble are absorbed more rapidly because they can readily cross cell membranes.
Cell membranes are composed of bilayers of lipids (fats) and phosphate “tails.” A common rule of chemistry is that “like dissolves like.” Because cell membranes are composed of lipids, a drug must be lipid soluble (lipophilic – “lipid loving”) to directly pass through a cell membrane. Some drugs are not lipophilic, rather they are hydrophilic (water loving) and cannot pass through cell membranes via direct penetration. Think about mixing water and oil. You can shake ajar of the two, but they will never dissolve into each other. They separate and settle into layers.
-
Facilitated Diffusion – This mechanism involves the passive movement of drug molecules across membranes with the help of specific carrier proteins. Facilitated diffusion does not require energy expenditure but is limited by the availability of carrier proteins and can become saturated at high drug concentrations.
-
Active Transport – In this process, drug molecules are transported across membranes against their concentration gradient with the help of carrier proteins and energy derived from ATP (adenosine triphosphate). This mechanism allows the absorption of drugs that are not easily absorbed by passive diffusion or are too large to pass through membrane pores.
Minimum Effective Concentration (MEC) refers to the lowest concentration of a drug in the bloodstream that is required to produce a desired therapeutic effect.
If the drug concentration falls below the MEC, the therapeutic effect may be insufficient or lost, leading to suboptimal treatment outcomes.
Toxic concentration refers to the level of a drug or substance in the bloodstream at which it causes harmful or adverse effects on the body. When the concentration of a drug exceeds the maximum safe concentration (MSC) and enters the toxic range, it can lead to undesirable side effects, organ damage, or even life-threatening situations.

A higher therapeutic index indicates a wider margin of safety, as there is a greater separation between the effective dose and the toxic dose. In other words, a drug with a high TI is considered safer because there is a lower risk of toxic effects at therapeutic doses.
Conversely, a drug with a low therapeutic index has a narrow margin of safety, meaning that even small changes in the dosing regimen or individual patient factors could lead to toxic effects.
Trough level – Also known as the minimum concentration, refers to the lowest concentration of a drug in the bloodstream, typically measured just before the administration of the next dose.
It is commonly used in therapeutic drug monitoring to ensure that drug concentrations remain within the therapeutic range, which is the range of drug concentrations associated with optimal efficacy and minimal side effects.
Monitoring trough levels is particularly important for drugs with a narrow therapeutic index, where small changes in drug concentrations can lead to significant differences in therapeutic effects or toxicity.
Eexamples of such drugs include
- vancomycin
- lithium
- digoxin
- warfarin
The half-life of a drug is a pharmacokinetic parameter that represents the time it takes for the concentration of the drug in the body to decrease by half. It helps healthcare professionals determine the appropriate dosing frequency and duration of treatment.
Half-life is influenced by two main processes: absorption and elimination. Absorption refers to how a drug enters the bloodstream, while elimination refers to the removal of the drug from the body, either through metabolism or excretion.
Several factors can affect a drug’s half-life, including:
-
Drug formulation: The formulation of a drug, such as immediate-release or extended-release tablets, can impact its absorption rate and thus its half-life.
-
Route of administration: The way a drug is administered (e.g., oral, intravenous, intramuscular) can affect its absorption and elimination, impacting the half-life.
-
Metabolism: The rate at which a drug is metabolized in the body can influence its half-life. This rate can vary among individuals due to factors such as genetics, age, and co-existing medical conditions.
-
Elimination: The efficiency of the body’s organs, such as the liver and kidneys, in eliminating a drug can affect its half-life. Factors like renal or hepatic impairment can significantly extend drug half-life.
Drugs with a short half-life may need to be administered more frequently to maintain their therapeutic effect, while those with a long half-life may require less frequent dosing.
Plateau drug levels, also known as steady-state concentrations, refer to the point at which the rate of drug administration (input) is equal to the rate of drug elimination (output) in the body.
At this stage, the drug concentration in the bloodstream remains relatively constant, fluctuating within a narrow range around an average value. Achieving plateau drug levels is crucial for maintaining consistent therapeutic effects during the course of treatment.
After repeated and regular administration of a drug, it typically takes about 4-5 half-lives for the drug to reach steady-state concentrations. The half-life of a drug is the time it takes for the drug’s concentration in the body to decrease by 50%. For drugs with short half-lives, plateau levels are reached relatively quickly, while drugs with long half-lives may take days or even weeks to reach steady-state concentrations.
In some cases, therapeutic drug monitoring may be employed to measure drug concentrations in the patient’s blood, allowing for adjustments in dosing to maintain plateau levels within the desired therapeutic range.
A loading dose is an initial, higher dose of a drug administered at the beginning of treatment to rapidly achieve the desired therapeutic concentration in the bloodstream.
This practice is particularly useful for drugs with long half-lives, as it can take a significant amount of time to reach steady-state concentrations with regular dosing alone. The loading dose helps to quickly establish an effective drug concentration, allowing the desired therapeutic effect to be achieved sooner.
After administering the loading dose, maintenance doses are given at regular intervals to maintain the therapeutic drug concentrations within the desired range.
A loading dose is an initial, higher dose of a drug administered at the beginning of treatment to rapidly achieve the desired therapeutic concentration in the bloodstream.
This practice is particularly useful for drugs with long half-lives, as it can take a significant amount of time to reach steady-state concentrations with regular dosing alone. The loading dose helps to quickly establish an effective drug concentration, allowing the desired therapeutic effect to be achieved sooner.
After administering the loading dose, maintenance doses are given at regular intervals to maintain the therapeutic drug concentrations within the desired range.
Courses and Games to Test Your Knowledge
Click on the corresponding images below to test your knowledge of the sympathetic and parasympathetic nervous systems. The first two options are mini courses that allow you to choose between flashcards, a randomized quiz, as well as line-’em-up and drag-and-drop interactions. The other options are games with rationales. You can use the menu on the right to jump to different subjects if you want to explore other topics. Learn by playing!
|
Sympathetic Nervous System [sc name=”kinetics1″][/sc]
|
Parasympathetic Nervous System [sc name=”dynamics1″][/sc]
|
|
Generic Drug Name Guess [sc name=”pyramid_generic_2″][/sc] |
Brand Drug Name Guess [sc name=”pyramid_brand_2″][/sc] |
|
Generic Name Word Guess [sc name=”wordguess_generic_2″][/sc] |
Brand Name Word Guess [sc name=”wordguess_brand_2″][/sc] |
|
Drug Class – Generic Name [sc name=”cardstack_generic_2″][/sc] |
Drug Class – Brand Name [sc name=”cardstack_brand_2″][/sc] |
|
Line It Up [sc name=”lineitup_2″][/sc] |
True or False [sc name=”true_or_false_2″][/sc] |




